We reproduce here the amazing paper published on November, 1998, by Dr. R. Balasubramaniam, who works at the Department of Materials and Metallurgical Engineering, Indian Institute of Technology (IIT). Dr. Balasubramaniam and other metallurgists at Kanpur IIT believe they have discovered a compound of Fe, O and H named Misawite protecting the cast Iron Pillar from rust.
To understand this paper in a better way it is important to note the difference between Barffing and Galvanizin processes: Barffing is coating some steel, not with zinc, but an oxide, protecting the surface projecting from acids or air or a drink. Through the Bower-Barff process, steel or iron is coated at high heat with iron oxide. Thus, this process is called Barffing. Besides, Galvanizing is coating iron or steel with zinc.
For further information to understand the preparation of gamma ferric oxide (gamma-Fe2O3) in suitable particles for use in magnetic media, please take a look at the following web site:
Now we transcribe the full article of Dr. Balasubramaniam:
THE CORROSION RESISTANT DELHI IRON PILLAR

The Delhi Iron Pillar is testimony to the high level of skill achieved by ancient Indian iron smiths in the extraction and processing of iron. The Iron Pillar at Delhi has attracted the attention of archaeologists and corrosion technologists as it has withstood corrosion for the last 1600 years. The several theories which have been proposed to explain its superior corrosion resistance can be broadly classified into two categories: the environmental and the material theories. Proponents of the environmental theories state that the mild climate of Delhi is responsible for the corrosion resistance of the Delhi Iron Pillar. It is known that the relative humidity at Delhi does not exceed 70% for significant periods of time in the year, which therefore results in very mild corrosion of the pillar.
On the other hand, several investigators have stressed the importance of the material of construction as the primary cause for the pillar's corrosion resistance. The ideas proposed in this regard are the relatively pure composition of the iron used, presence of Phosphorus (P) and absence of Sulphur/Magnesium in the iron, its slag-enveloped metal grain structure, and passivity enhancement in the presence of slag particles.
Other theories to explain the corrosion resistance are also to be found in the literature like the mass metal effect, initial exposure to an alkaline and ammoniacal environment, residual stresses resulting from the surface finishing operation, freedom from sulphur contamination both in the metal and in the air, and surface coatings provided to the pillar after manufacture (barffing and slag coating) and during use (coating with clarified butter).
That the material of construction may be the important factor in determining the corrosion resistance of ancient Indian iron is attested by the presence of ancient massive iron objects located in areas where the relative humidity is high for significant periods in the year (for example, the iron beams in the Surya temple at Konarak in coastal Orissa and the iron pillar at Mookambika temple at Kollur situated in the Kodachadri Hills on the western coast). It is, therefore, obvious that the ancient Indians, especially from the time of the Guptas (300-500 AD), produced iron that was capable of withstanding corrosion. This is primarily due to the high P content of the iron produced during these times. The addition of P was intentional as iron produced during earlier times does not show the presence of P.
To understand the precise reason for the corrosion resistance of the Delhi Iron Pillar, we analysed the composition of the rust on a Gupta period corrosion resistant iron clamp and also the rust on the Delhi Iron Pillar. Archaeometallurgical studies form a small component of our research activities. It is clear that referring to the Delhi iron pillar as rust-less is misleading as the iron pillar derives its corrosion resistance from the passive surface film (i.e. rust) that forms on the surface. We undertook a detailed rust analysis using modern sophisticated characterization techniques like Mössbauer spectroscopy and Fourier transform infrared spectroscopy (FTIR). We summarize below some of the exciting results of our study. The present study also provides valuable insight into the corrosion resistance of steels.
Microstructure
The microstructure of the iron of the Delhi iron pillar is typical of wrought iron. Iron was produced in ancient times by solid-state reduction of iron ore using charcoal and after the reduction process, the slag particles in iron were squeezed out by hammering. This invariably resulted in the presence of slag particles and unreduced iron oxide in the microstructure. We have earlier shown by theoretical mixed potential analysis and experimental potentiodynamic polarization studies (conducted on ancient iron) that the presence of slag particles could enhance passivity in these ancient irons containing P. However, the role of P in the passivation process was not understood. The characterization of the Delhi iron pillar rust has provided clear ideas about the passive film formation process on the Delhi iron pillar.
Rust Analysis
The FTIR spectrum proved the presence of g-FeOOH, a-FeOOH and d-FeOOH. The d-FeOOH was the major component of the rust as the peak was of relatively larger height compared to the others. An interesting result from the FTIR spectrum was that there was a distinct signal from the phase FePO4.2H2O and the shoulder from this phase was also identifiable. Therefore, the results of the FTIR study indicated that the constituents of the scale were g, a and d-FeOOH, in addition to a small amount of FePO4. In order to further understand the nature of the rust, the Mössbauer spectrum obtained from the rust in the transmission mode was analysed. The presence of g-FeOOH, a-FeOOH and d-FeOOH in superparamagnetic form was confirmed. The very fine particle size of these oxyhydroxides was also confirmed. The presence of iron phosphate was also confirmed. Finally, the rust was also composed of magnetite that was incorporated with some ions.
SURFACE FILM CAHARACTERISTICS
Process of Protective Rust Formation
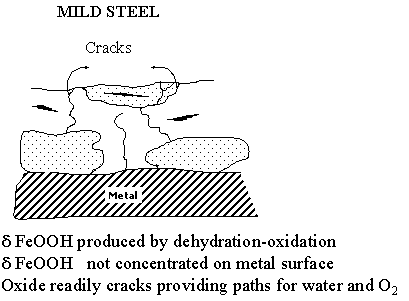
The process of protective rust formation on the ancient Indian iron clamp can now be outlined based on the results presented above. The surface film characteristics of the Delhi iron pillar has been compared with that of mild steel in the accompanying figure. The rusting of normal mild steel and weathering steel is first addressed. When iron is exposed to the environment, the first oxides that form are the oxyhydroxides of Fe which are oxidized from Fe(II) complexes. Although several different allotropic modifications of the oxyhydroxides have been proposed to form on the surface of iron on initial exposure to the environment, there is firm evidence in the literature to suggest and prove that the first oxyhydroxide to form is g-FeOOH. After this is formed, a part of it begins to transform to another allotropic modification (a-FeOOH) and the rust at later times is composed of both these oxyhydroxides. These oxyhydroxides are not protective against corrosion and they readily crack allowing for ingress of oxygen and moisture to reach the metal surface and cause further corrosion. However, with time, a part of the FeOOH formed transforms to magnetic oxides of iron, which are much more protective than these oxyhydroxides. Mössbauer studies of rust formed on steel exposed to the environment clearly shows that Fe3O4 (more precisely to be called Fe3-xO4) forms first and this is later converted to g-Fe2O3. The formation of this magnetic oxide results in protection and the oxidation (corrosion) rates decrease once these oxides form on the surface from the oxyhydroxides. In addition to a- and g-FeOOH, there can be another oxyhydroxide d-FeOOH which can form on atmospheric exposure of iron.
It is interesting to note that d-FeOOH is generally amorphous in nature. In ordinary mild steels, this phase does not form as a continuous layer but rather in a discontinuous manner as it results due to dehydration-oxidation of the Fe(II) complexes. Therefore, the d-FeOOH that forms in ordinary mild steels is not protective in nature. However, it is possible for this d-FeOOH to form next to the metal surface as a continuous layer in which case the steel obtains corrosion resistance, as the oxyhydroxide is also amorphous in nature. The formation of d-FeOOH as a continuous layer next to the metal surface is catalysed by the presence of P (also Copper [Cu] and Chromium [Cr]) in the material. Moreover, the d-FeOOH is enriched with P and other elements that are added for improving atmospheric corrosion resistance like Cr and Cu. The presence of this amorphous layer is the reason for the excellent corrosion resistance of the so-called weathering steels.
In the case of ancient Indian iron, the atmospheric corrosion rate of the matrix material would be accelerated initially, in the presence of slag particles, leading to the enhancement of P concentration near the surface. Corrosion rate measurements (by Tafel extrapolation and weight loss methods) indicate that the short term corrosion rate of ancient Indian iron is an order of magnitude higher than that of 0.05%C mild steel in acidic environment while it is comparable in mildly alkaline environment. It must be noted that these measurements were obtained for complete immersion conditions, quite different from atmospheric exposure. Nevertheless, the initial corrosion of the matrix must lead to enrichment of P content near the surface. This is verified by compositional analysis of the metal next to the oxide which indicated enrichment of P in these regions. With the enhancement in the P concentration, the formation of d-FeOOH is catalysed and it should form as an amorphous compact layer next to the metal surface. Therefore, it appears that the presence of a significant amount of P is crucial to the corrosion resistance of the ancient Indian iron.
The process of passive film formation on the ancient Indian iron can be visualized as follows. Initially, the corrosion of the metal leads to the formation of a- and g-FeOOH. However, the presence of slag particles accelerates the corrosion of iron thereby enhancing the P concentration on the surface. This enhancement of P on the surface catalyses the formation of amorphous d-FeOOH as a compact layer next to the surface and this results in atmospheric corrosion resistance of the Delhi iron pillar. With time, conversion of this d-FeOOH to a stable form of iron oxide, i.e., magnetite, is possible. The magnetite could be doped with ions. This would further enhance the corrosion resistance of the surface film on the surface. The FTIR and Mössbauer spectra indicate the presence of iron phosphates. The presence of these phosphates would provide further corrosion resistance to the passive film by lowering ionic diffusion in the oxide and also by blocking the pores in the oxide. The golden hue of the pillar when viewed in certain orientations is due to the presence of iron phosphates. We hope to compositionally map the rust on the entire exposed surface of the pillar in the near future.
For more information contact:
Dr. R. Balasubramaniam
Department of Materials and Metallurgical Engineering
IIT Kanpur, Kanpur 208016
Telephone: (0512) 59 7089
e-mail: bala@iitk.ac.in